

Start-up medical device companies on a fast-track plan to implement their ISO 13485:2016 quality management system should include planning for the fast tracking of the final process step, which is the certification of their implemented QMS by a recognized, accredited ISO 13485 certification body. This short article explains the certification process and although it may vary slightly depending on the certification body the general steps will be the same.
The diagram below shows the basic steps in the QMS implementation process, and a breakdown of the steps involved in the certification process.
Implementation and Certification process
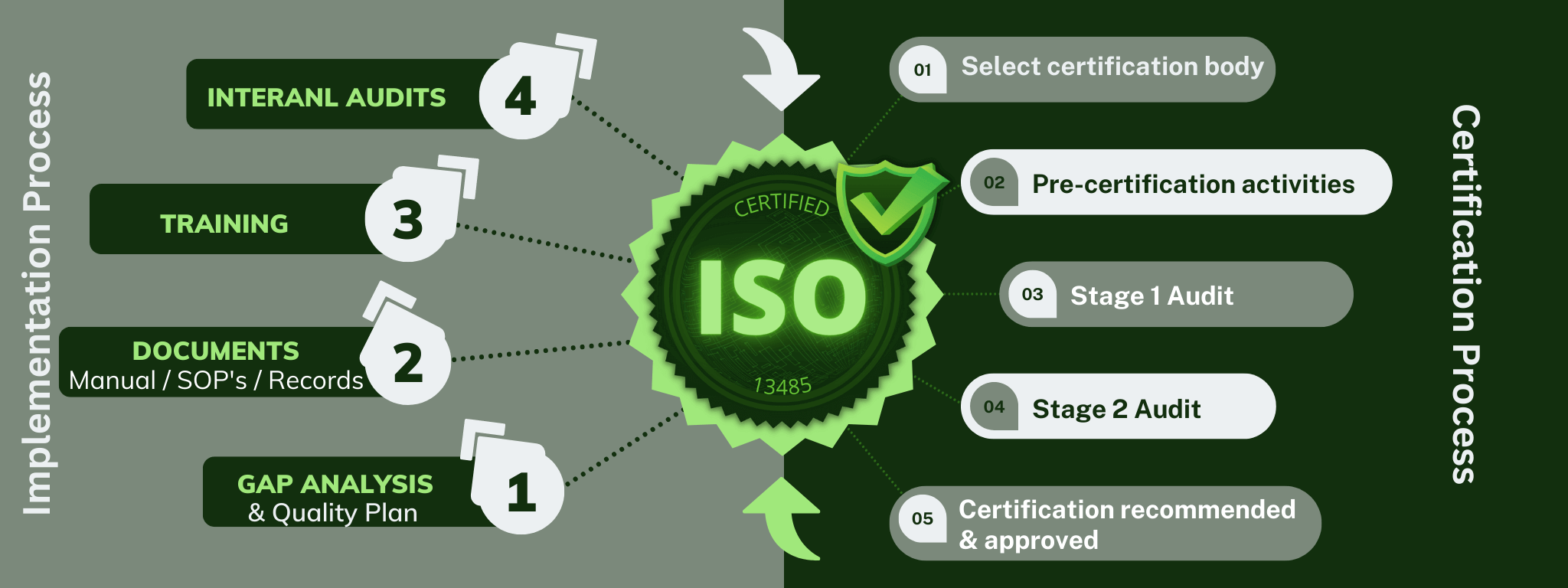
What you should complete in your QMS implementation before your begin the certification process:
In order for time efficiency and staying on your fast-track implementation and certification plan you should have the following completed before beginning the formal certification process:
- Have a clear understanding of the standard and its requirements
- Completed the implementation of your ISO 13485:2016 quality management system
- Meet all the appropriate statutory and regulatory requirements
- Training of employees
- Site and facilities in good shape and ready for inspection
- Have records in place as evidence of compliance to your QMS procedures
- Completed Internal Audits of your QMS
- Completed Management Review(s)
You can select your certification body and make initial contact with them before your 100% complete on the above but once you submit your certification process application the first assessment will need to be completed within 90 days.
The steps to ISO 13485:2016 Certification:
1) Preliminary assessment (Optional):
This is a voluntary activity offered by the leading certification bodies and is performed prior to the formal assessment steps. It can include a gap analysis between your implemented quality management system and the requirements of the standard.
I highly recommend you include this assessment once you?re ready, and it can provide good guidance and feedback on your readiness for the audits and in my experience can be a very positive first step in the certification process.
It is normally conducted on-site and includes an assessment of your facility, processes and quality management system. The result can be a gap analysis in compliance to the standard requirements and provide you with the potential risks and opportunities for improvement.
Based on the status of your readiness and the time expected to complete any gaps, the formal audit assessment activities can be planned and scheduled.
2) Stage 1 Audit:
Normally within 90 days of scheduling your Stage 1 audit the audit will take place. For fast-tracking your implementation completion and certification this should be done sooner than 90 days and all the more reason to be in good shape with your complete quality system and any known issues resolved.
On completion of the audit a Stage 1 Audit report will be issued including general observations as well as any minor and major nonconformities.
Minor nonconformities:
So long as there are not too many minor nonconformities, they should not be enough to hold up the scheduling of the Stage 2 audit and can be resolved internally using your CAPA procedure and will be reviewed by the auditors during the Stage 2 audit.
Examples of minor nonconformities could include:
- Missing a training record
- Scheduled holding of internal audit delayed
- Required attendees for Management Review absent
- Calibration schedule for single measuring device delayed
Major nonconformities:
Any major nonconformities will need to be corrected through your CAPA procedure and submitted and approved by the certification body before you can procced to the Stage 2 audit causing possible delays in your certification.
Any major findings will also be a focus of attention of the auditors during your next audit, so your corrective actions need to be robust and include preventative actions to provide high confidence of no repeats.
Examples of major nonconformities could include:
- Corrective actions completed without following all steps required by the procedure
- Critical to quality process changes made without validation
- Deviations to product specifications not approved
- Labeling design changes made without following design control procedures.
3) Stage 2 Audit:
After the successful completion of your Stage 1 audit the Stage 2 audit can be carried out to confirm your quality management system is fully implemented, in use and effective, and conforms to the requirements of the ISO 13485:2016 standard.
The Stage 2 audit will include:
- Looking at evidence for following elements of the standard and your procedures
- Verifying that your quality management system complies with the appropriate legal and regulatory requirements.
- Evaluating the overall effectiveness of your quality management system
- Requiring you to show how you follow the monitoring, measuring, reporting and analyzing the QMS against key quality objectives
- Ensure that you have conducted Internal Audits and Management Reviews
- Evaluation of the controls for your manufacturing processes
- Looking closely at you customer complaint handling and corrective action process
- Document controls and records
- Training
If all looks good with only some minor findings, then the recommendation will be submitted for your formal certification. However if there are any major findings these will need to be resolved before a certificate can be issued and may require another visit from the auditors to check the corrective action for the findings.
4) Certification:
On completion of the Stage 2 audit and resolution of any major findings the certification body will recommend your company to the compliance team for final approval. If you only have a few minor findings and you have a corrective action procedure in place this should not be a problem and a delay in your certification.
Time to celebrate your well-earned ISO 13485 Certification
Once the certificate is awarded it is good for 3 years and normal practice is for the certification body to conduct annual surveillance audits with perhaps the first such audit 6 months after your certification.
?????Use Fast-Track QMS Consultants to help plan and achieve your ISO 13485:2016 Implementation and Certification
Helping start-up medical device companies fast-track their implementation of their ISO 13485:2016 Quality Management System and obtain their Certification is what we do. We have the products, documentation templates for procedures, forms and a quality manual package. Also services including training, consulting, and auditing, that can save you significant time and provide you with the confidence that you will achieve certification on plan.
Take a look at these articles to further guide you in your QMS journey
???????????????????
