

If you are a start-up medical device company, you are most likely focused full time on working to develop and bring to market your product as quickly as possible. You may not have the time, or experienced quality and regulatory resources to implement a complete ISO 13485:2016 quality system and obtain certification.
Here's the problem - If you don't have a complete quality system in place, you will not be able to obtain ISO 13485:2016 certification and without certification you will be limited on the markets for your medical device products.
This article outlines the steps for a planned ISO 13485:2016 implementation, a summary of the standards requirements, and how Fast-Track QMS Consultants can help.
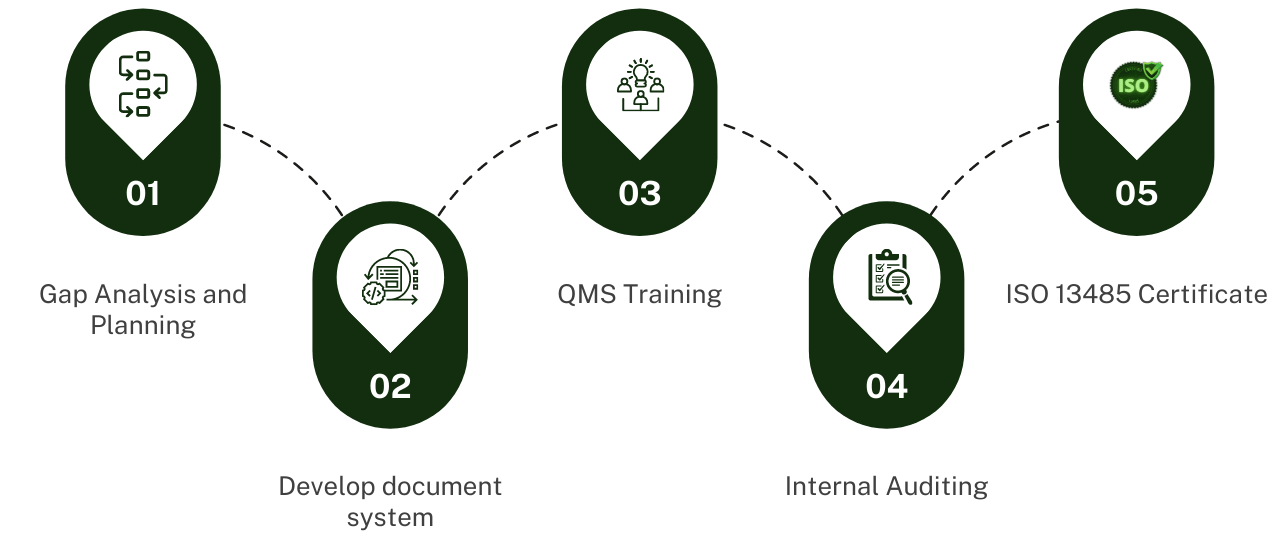
First let?s look at the basic 5 activities in a well-managed quality management system implementation. This is a simplified illustration, as obviously the planning should come first, and the certification will be the final step, but for the others they can and should, overlap as required. For example, training should start with the ISO 13485:2016 standard for key employees and management and continue for procedures as they are developed and before release. Auditing should also start as the documented procedures are implemented to ensure compliance, and make any improvements as required. Finally, initiating the certification process with the selection of a certification body should start at the appropriate time.
5 Key Activities In The Implementation of Your QMS:
Before looking at these 5 steps, here is a summary of the elements included in ISO 13485:2016 and a sample of the some of the required procedures: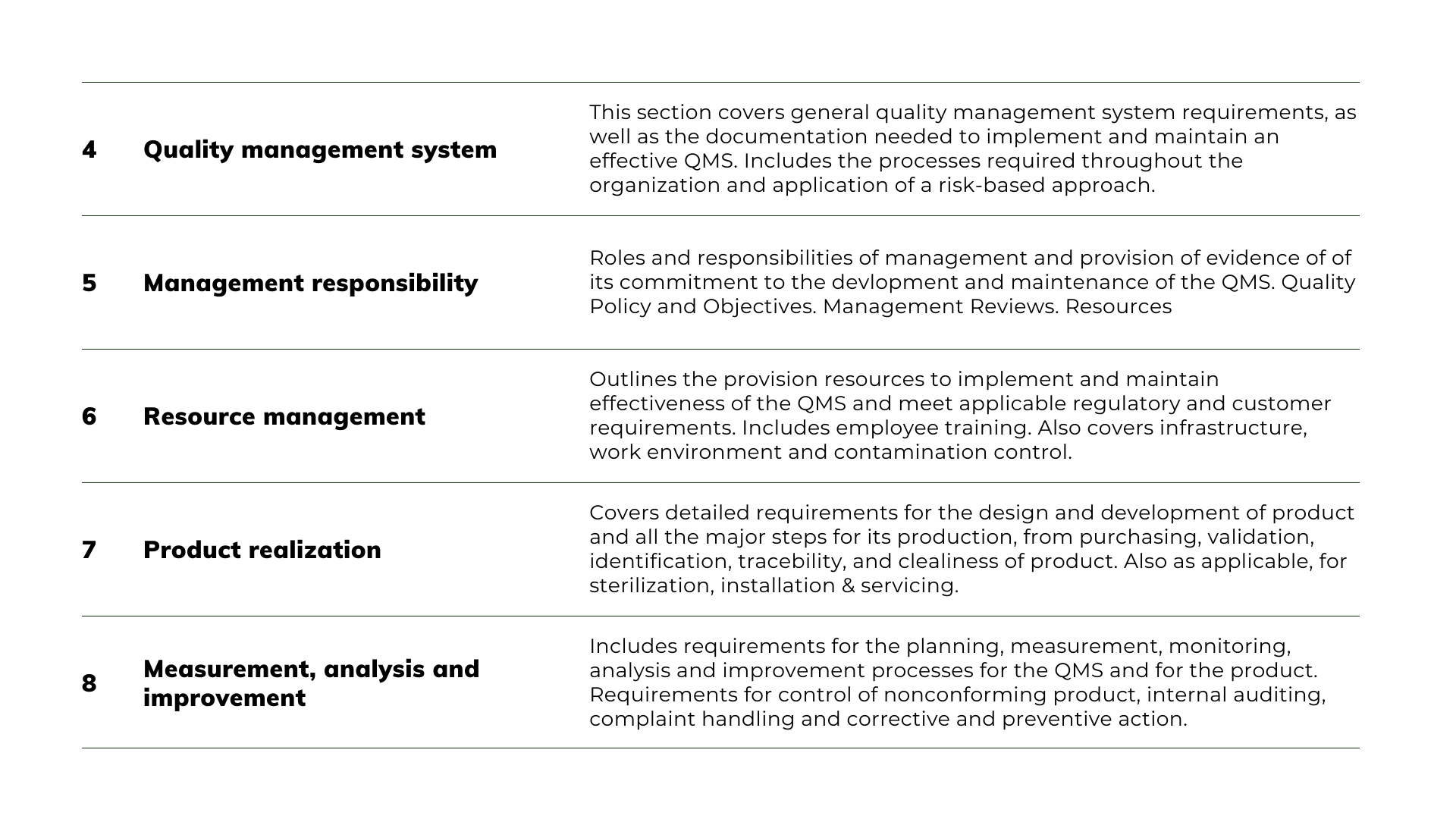
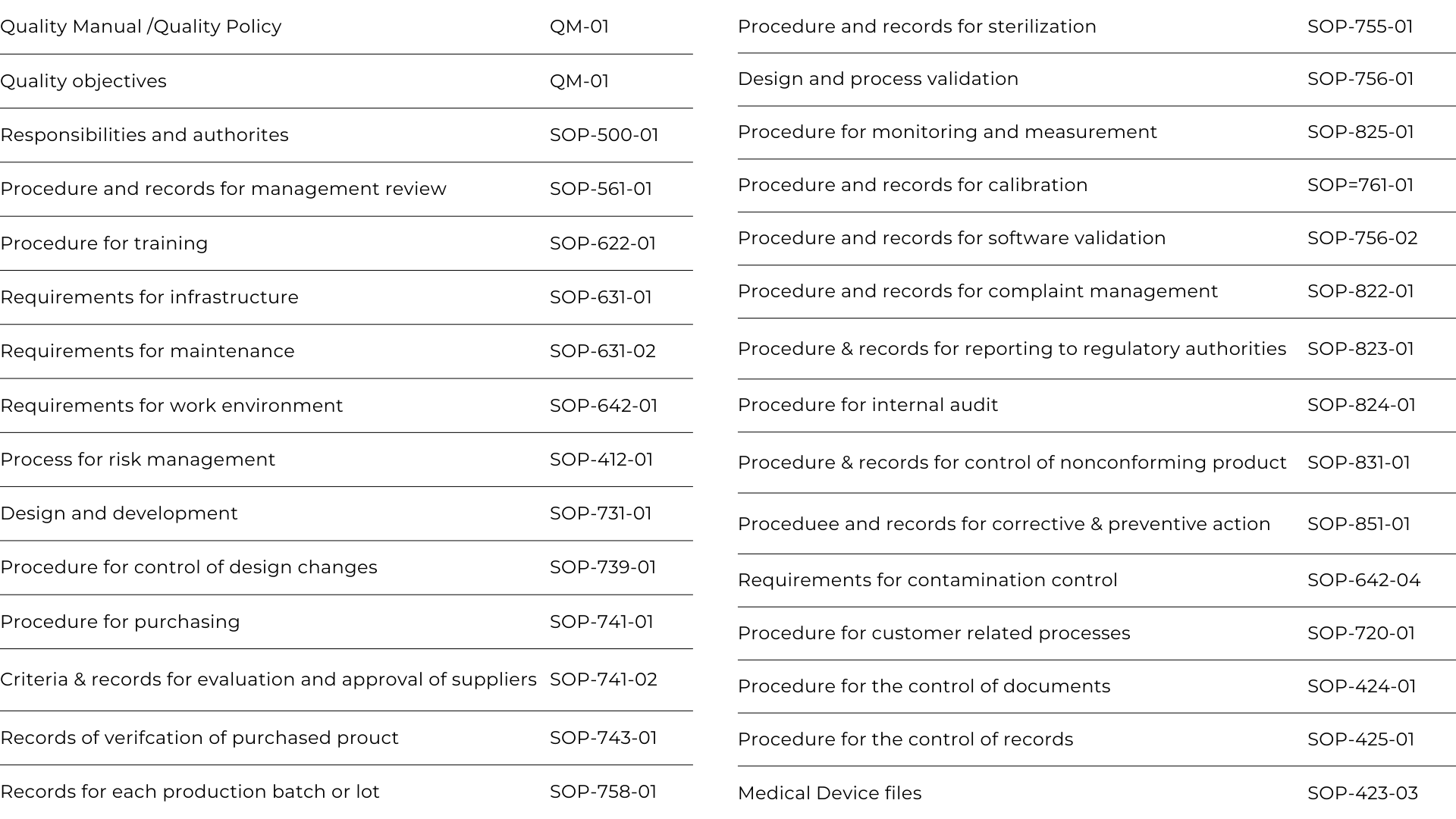
Note: The above documents shown are only a sample of the required procedures and typically, and depending on the market requirements, most ISO 13485:2016 quality systems will have around 50 procedures
???????????????????????????????????????????????????????
Now let?s look at each of the 5 activities for ISO 13485:2016 Implementation and Certification:
1) Gap Analysis and Planning:
Planning for your ISO 13485:2016 implementation and certification is a critical first step if you want to fast-track your implementation and achieve certification on plan.
Also, if you?re a start-up company you should understand right up front what the detailed requirements are to meet the ISO 13485:2016 standard. Then complete a gap analysis and implementation plan based on the current status of your quality management system and where you need to be in order to obtain certification. This is the right way to start your project and one I highly recommend.
Your planning also needs to consider the resources you have and the expertise and experience of those resources. If this is an area of concern, you may want to consider using a consultant to help with your implementation. Many start-up medical device companies may not have the experienced resources to implement the quality system, or if they do, they may be tied up with supporting other start-up tasks such as helping the design and development of the medical device, validations of the manufacturing facilities, regulatory requirements, etc.
Planning your implementation has many variables to consider, including:
- Are you starting your QMS from scratch or do you have some basic system in place?
- At what stage of development is your medical device and manufacturing capabilities?
- Are resources available , are they trained or have experience with ISO 13485:2016
- What are the intended markets for your medical device, regulatory requirements, etc.
 Fast-Track QMS Consultants can help start-up medical device companies plan your ISO 13485 implementation and certification and from our website we offer free of charge a Fast-Track Planning kit including a Gap Analysis template, Power Point Training on ISO 13485:2016 and Guidance Manual on Implementation Planning. Plus, a 30- minute online consulting call.
Fast-Track QMS Consultants can help start-up medical device companies plan your ISO 13485 implementation and certification and from our website we offer free of charge a Fast-Track Planning kit including a Gap Analysis template, Power Point Training on ISO 13485:2016 and Guidance Manual on Implementation Planning. Plus, a 30- minute online consulting call.
You can download your FAST TRACK PLANNING KIT HERE
2) Develop Document System:
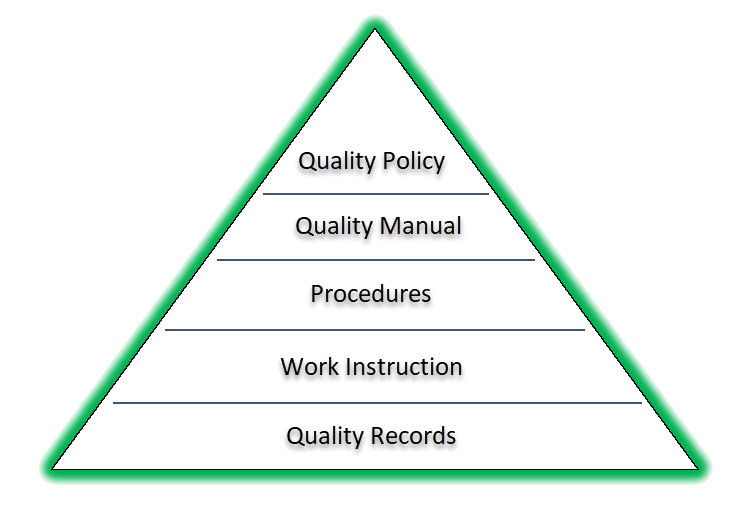
Typically quality system documentation is represented in a pyramid as shown below and although can vary slightly depending on the structure of your quality system it is essentially showing the documentation required.
Creating this documentation as part of your ISO 13485:2016 implementation is a significant amount of work and can take many months to develop, conduct training, release, control, audit, update and maintain records to demonstrate compliance.
The documentation should not be overly burdensome, be clear and functional to represent the requirements of the standard, your medical device, the markets you?re in, regulatory requirements and of course to control the quality and safety of your product
Establishing your document system, conducting the required training and overall control of the document system, is one of the most important foundational elements of a quality management system. This should be managed and guided by an experienced quality professional, and ideally someone with experience and knowledge of the ISO 13485 standard.
There is software available specifically designed for medical device quality systems that can make managing the control and use of your documentation and general QMS a lot easier. It can include document control as well as design controls, CAPA management, supplier management, nonconformance, audit management, and customer complaint management.
Fast-Track QMS Consultants can provide guidance on the selection of the right software to meet the needs of your medical device quality management system.
QMS Document Structure
Quality Manual:
A well designed and written Quality Manual should be easy to read and clearly correspond to the elements of the ISO 13485 standard, as well as describe the companies quality management system. It serves as the foundation of the documented quality system and is a requirement of ISO 13485:2016, and most other quality management systems. The US FDA is currently an exception where it is not a documented requirement but expect this to change as they head towards adopting the ISO 13485 Standard.
In basic terms the Quality Manual can be considered as the user?s guide to your QMS and is a good reference for your management and employees. It is also where external auditors will start reviewing your QMS. It needs to be easy to read and cover the applicable requirements of ISO 13485:2016
Your Quality Manual is required to include the following:
??????????????????????????????????????????
- The scope of your quality management system, plus any exclusions along with justification
- The structure of your QMS documentation (see diagram shown in item 2 above)
- The procedures that make up your QMS, and can be a reference or as attachment
- Outline of the QMS processes and their interactions
The Quality Manual can also include, and in my experience is good to do so rather than having separate documents:
- Quality Policy, and the Quality Manual is a great location to document your Quality Policy
- Applicable Standards and Regulations: list those applicable to your markets.
- Definitions: and abbreviations used in your manual
- Cross Reference Addendum: as already mentioned it is more efficient to have QMS procedures referenced in a separate document rather than in
your Quality Manual, although either way is ok but listing in an attachment is I have found easier to navigate and find the appropriate procedure.
 Fast-Track QMS Consultants can provide a Quality Manual bundle which includes a 35-page Quality Manual template along with supporting documents:
Fast-Track QMS Consultants can provide a Quality Manual bundle which includes a 35-page Quality Manual template along with supporting documents:
More detailed information HERE
????????????????????????????????Procedures:
Standard Operating Procedures (SOP?s) are a requirement of the elements of the ISO 13485:2016 Standard (sample of these shown earlier), and along with other documents and records are the backbone of your quality management system.
They should be written in a concise and clear format and follow a standard structure:
- Purpose
- Scope
- Responsibilities
- Definitions
- Procedure
- Reference Documents
For Fast-Track QMS Consultants advise on which procedures to implement as a priority and to fast-track your plan for your QMS implementation, click the links below guides to help you further on your journey.
For document control each procedure needs to include a procedure title, number, activated date, and revision history.
For a start-up medical device company writing a Quality Manual and up to 50+ SOP?s, can be a real challenge, and especially if they have limited resources who may not have experience with ISO 13485:2016. One solution is to use an outside consultant and Fast-Track QMS Consultants have the experience and the document templates to help start-up medical device companies with the implementation of their quality management system. One major product offering is the Turnkey Quality System Bundle shown below:
 The Turnkey Quality System bundle includes:
The Turnkey Quality System bundle includes:
- Quality Manual template and supporting documents
- 50 Procedures covering all elements of ISO 13485:2016, plus FDA 21 CFR 820 and MDR
- 41 Forms that support the procedures
- 10 -hours of on-line consultation at no charge
More detailed information HERE
?????????????????????????????????????3) QMS Training:
Training needs to start early in the implementation of the quality system, with training on the ISO 13485:2016 standard for those involved in the implementation, and for senior management. Training then continues throughout the implementation process as procedures are developed, released, and updated, and as the manufacturing processes are implemented.
Important elements of QMS training include:
- Training requirements for each procedure and job position, e.g. training matrix
- Establishing a training plan
- Training for new employees before they start their work responsibilities
- As appropriate, establish training effectiveness
- Employee awareness of potential quality issues if the activity is not performed properly
- Training records
4) Internal Auditing:
Internal audits should be looked at as a means for identifying gaps and improvement opportunities for your quality management system. This is true even for well established medical device companies and is especially true for start-up companies, that for sure are going to have gaps as they progress through their implementation of ISO 13485:2016.
My advice based on my experience is don?t wait until you have completed your QMS implementation and then check the box by conducting the internal audit just before the scheduled certification audits. This can cause major delays as your rush to make corrections and could risk your fast-track plan.
Start auditing early in your implementation process. Can at first be focused on auditing compliance with your first released procedures. This then provides the opportunity to make ongoing improvements rather than all the required changes piling up later.
Use a risk-based approach to your audit planning and schedule your audits throughout the implementation cycle.
Document the audits and use corrective and preventive actions procedures as appropriate to make improvements and close the gaps.
?????????????????????????????????????????????????
Finally and very important is to have your auditor?s trained in the ISO 13485:2016 quality system requirements and especially your lead auditor should be qualified as such.
5) ISO 13485 Certification:
To maintain a fast-track plan for the final step of your ISO 13485:2016 implementation and certification you need to start the certification process by selecting a certification body and making initial contact with them during the final stages of your total QMS implementation and once you have the following close to completion:
- Have a clear understanding of the standard and its requirements
- Implementation of your ISO 13485:2016 quality management system
- Meet all the appropriate statutory and regulatory requirements
- Training of employees
- Site and facilities in good shape and ready for inspection
- Have records in place as evidence of compliance to your QMS procedures
- Completed Internal Audits of your QMS, and held documented Management Review(s)
The diagram below outlines the steps involved in the certification process and can take several months to complete:
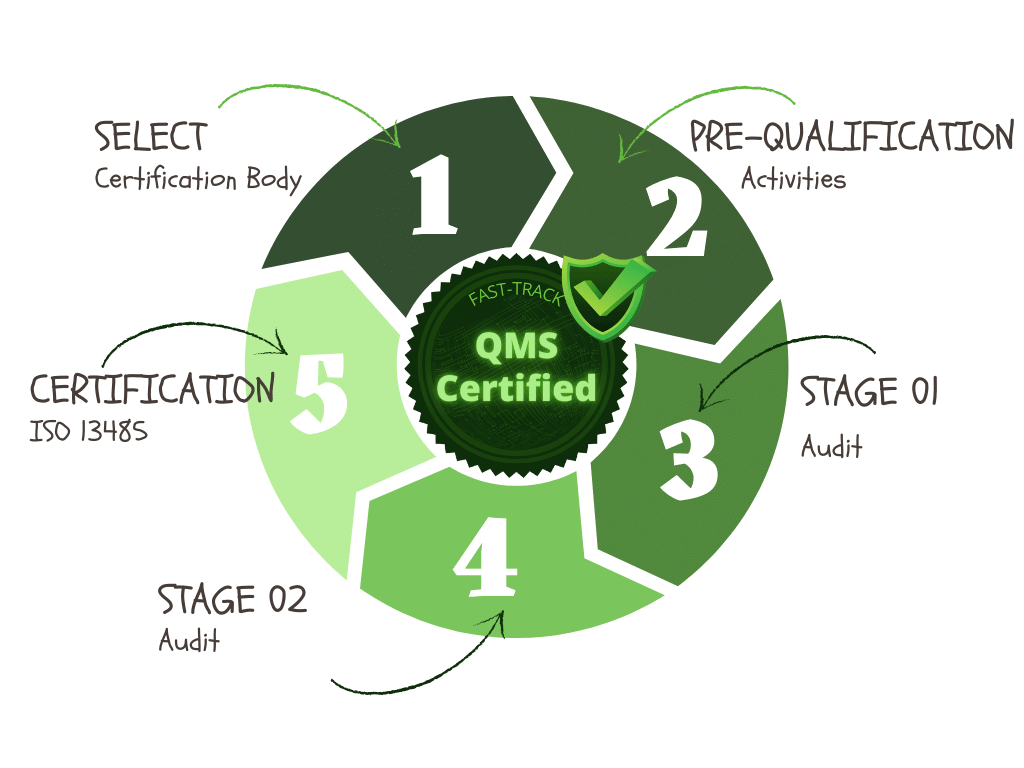
Pre-Certification activities:
I highly recommend this voluntary step for a start-up medical device company. It includes assessment of your facility, processes and QMS by the certification body. The result can be a gap analysis for compliance to the ISO 13485:2016 standard requirements, provide you with the potential risks and opportunities for improvement, and helps you to be prepared for the formal audit process.
Stage 1 Audit:
This will normally occur within 90 days of scheduling your Stage 1 audit.
For fast-tracking your implementation completion and certification this should be done sooner than 90 days ,and all the more reason to be in good shape with your complete quality system
and any known issues resolved.
On completion of the audit a Stage 1 Audit report will be issued including general observations
as well as any minor and major nonconformities. Any major nonconformities will need to be corrected through your CAPA procedure before you can procced to the Stage 2 audit.
Stage 2 Audit:
After the successful completion of your Stage 1 audit the Stage 2 audit can be scheduled and carried out to confirm that your quality management system is fully implemented, in use and effective, and conforms to the requirements of the ISO 13485:2016 standard.
The Stage 2 audit will include:
- Looking at evidence for conformance to the elements of the standard and your procedures
- Verifying that your QMS complies with the appropriate legal and regulatory requirements.
- Evaluating the overall effectiveness of your quality management system
- Ensure that you have conducted Internal Audits and Management Reviews
- Evaluation of the controls for your manufacturing processes
- Looking closely at you customer complaint handling and corrective action process
- Reviewing your QMS documents and document and records controls
If all looks good with only some minor findings, then the recommendation will be submitted for your formal certification. However if there are any major findings these will need to be resolved before a certificate can be issued.
Certification:
On completion of the Stage 2 audit and resolution of any major findings the certification body will recommend your company to the compliance team for final approval. If you only have a few minor findings and you have a corrective action procedure in place this should not be a problem and delay your certification.
For more detail on Planning for your Fast-Track Certification and the 5 steps involved check out our blog by clicking HERE.
Once the certificate is awarded it is good for 3 years and normal practice is for the certification body to conduct annual surveillance audits. The first audit can be 6 months after your certification.
Final thoughts and a summary of how Fast-Track Consultants can assist with your ISO 13485 Implementation and Certification:
For a start-up medical device company focused on developing their medical device and manufacturing capabilities, plus all the other associated tasks, the development, implementation, and certification of an ISO 13485:2016 quality management system, can be a monumental task.
I have found with many companies, this process if conducted with limited internal resources who may not have previous experience with ISO 13485:2016 and are often also involved in supporting the other priorities to support the launch, can take 12 to 18 months.
There is also a greater risk, if limited available experienced resources, that errors will be occur, and gaps maybe found late in the implementation and certification process that cause further delays and missed target certification dates. This can lead to delayed product launch dates and longer time to reach financial payback.
Any gaps in the quality system can also contribute to product quality and safety issues which can also be costly and time consuming.
So for a start-up medical device company you can spend the time and effort to find and hire the quality team you will need, or you can hire an experienced consultant, or even a combination of both of these options.
???????????????????????????????????
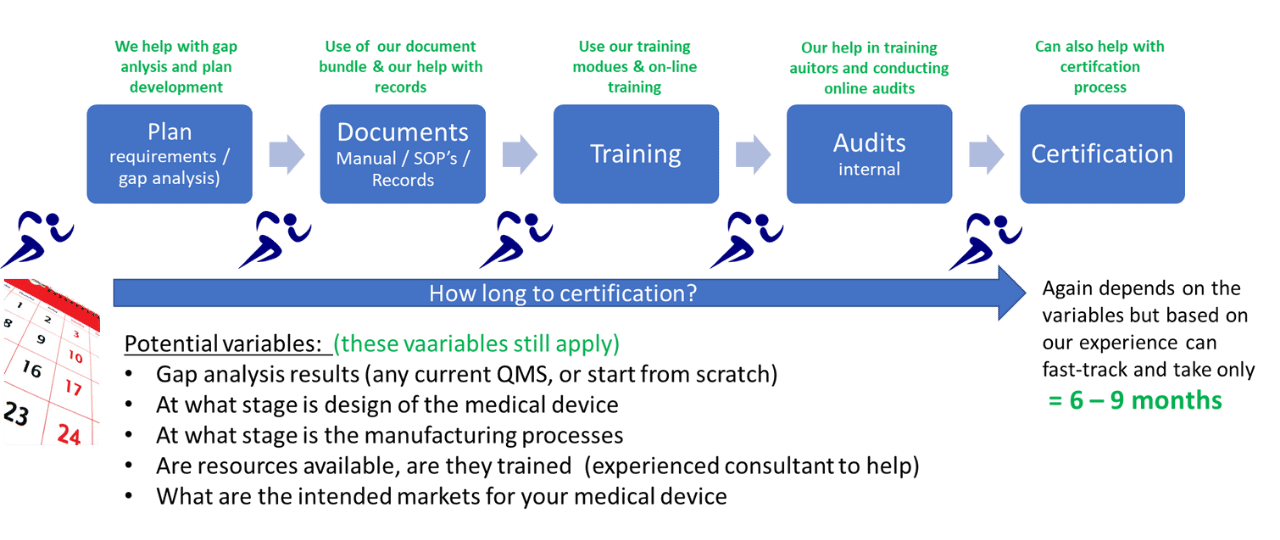
The products and consulting services we can provide:
1) Gap Analysis and Planning:
Our Free Fast-Track Implementation Kit is intended for medical device businesses that have not yet started or are in the early stages of the planning and implementation of their ISO 13485:2016 Quality System. See page 3 for more on implementation planning and gap analysis and the planning kit we offer.
2) Develop Document System:
This is where we can provide a major benefit and time savings to your fast-track implementation plan with our Turnkey Quality System Bundle or just our Quality Manual Bundle. See previous pages for more on this.
The documents contained in these bundles are all written in MS Word for easy customization to suit your specific requirements. They are written by experts in ISO 13485:2016 and have been proven over time and many, many, audits. They cover all the elements of ISO 13485:2016 as well as meeting requirements of FDA CFR 820
3) QMS Training:
Fast-Track QMS Consultants can offer training materials for elements of ISO 13485:2016 for start-up companies who want to conduct the training themselves, or we can assist with online training.
4) Internal Auditing:
We have trained and experienced quality system auditors who can assist with your internal audits, and/or train your auditors, and as required help with any corrective actions from the audits.
5) Certification:
Fast-Track QMS Consultants can also help with the certification process. This can include recommendations for selection of a globally recognized certification body, preparation and readiness for the certification process, and finally any help that may be required to support the certification process itself.
