If you?re a start-up medical device company, planning on implementing ISO 13485:2016 and you need to get this done and certification completed as fast as possible so you can start getting a return on your investment, then this short article might be right for you.
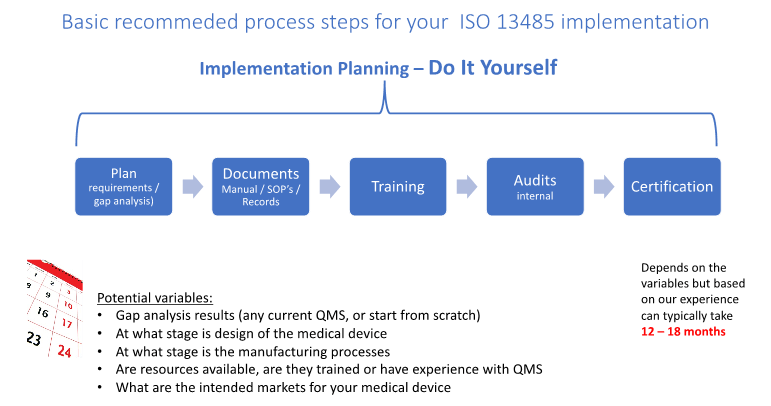
Even if you are an established company with a quality system in place but need to move into a new market which requires you to have certification for ISO 13485 these steps can still be appropriate. The diagram below shows the basic steps for implementation planning and assumes you?re going to plan and implement with existing resources.
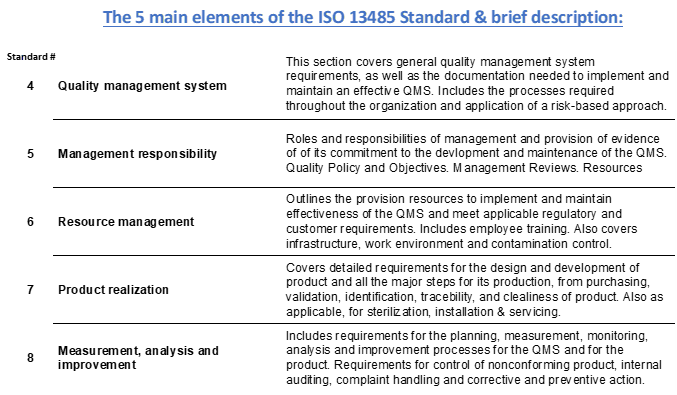
Before we get into how this timeline can be reduced significantly, and also provide a proven quality system, as well as high confidence on successful certification, here is a brief outline of the requirements for ISO 13485:2016:
The mandatory procedures shown are not all that is normally required and typically the total number of procedures is closer to 50 SOP?s.
????????????????????????????????
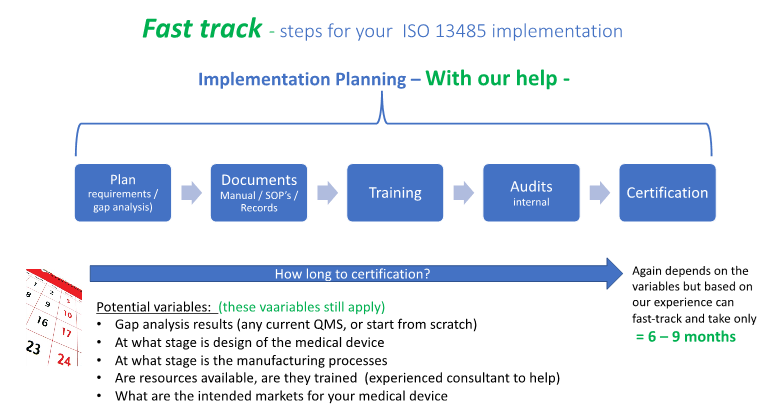
The diagram below shows how using Fast-Track QMS Consultants can help your ISO 13485 implementation all the way from the gap analysis and planning steps, use of our proven SOP templates and Quality Manual, plus to suite your needs, training and consulting service to reduce your timeline to
6 ? 9 months.
These are the same steps required for a good implementation plan if you carry out internally with your own resources or if you use an experienced consultant to help you fast-track the process. The difference though can be substantial as far as time savings and providing confidence in meeting the timeline and obtaining certification on plan. The use of the available documentation templates, training materials, auditing and general consultation can be your fast-track road to success.
Here is a little more on what is available from our website:
On our website you can download, at no charge, our gap analysis template and/or the full implementation planning kit which includes:
????????????????????????????????????
1) PowerPoint presentation ? Understanding ISO 13485:2016 and how to plan for its fast-track implementation
2) Gap Analysis check sheet
3) Guidance Manual - Fast Tracking your QMS Implementation and Certification
4) Includes also a 30-minute phone or vCall for consultation on completing the gap analysis and planning steps. More time can be made available if required.
2. Documentation:
Also from our website you can purchase the Quality Manual bundle, or the complete Turnkey documentation bundle which includes the Quality Manual and complete set of procedure templates and forms that cover all elements of ISO 13485.
The Turnkey Documentation bundle also includes 10 hours of free consultation to help with the implementation as required. Heck out the website for more details.
3. Training:
We have training materials available and can also provide online training as required.
We can conduct training for your internal auditors and assist with auditing as required.
5. Certification:
The selection of a globally recognized certification body is a key step, and we can help with the selection based on our experience and also help with the certification process as required. The timing of starting the certification process is critical to the success of completing your implementation and certification on plan, and of course your product launch. Too soon and before you have documentation including records and completed, some internal auditing done
Summary:
This was summary of the planning steps that should be followed for a start-up medical device company or for an established business that wants to fast-track their implementation of ISO 13485:2016. Planning does not need to take a lot of time but it is a very important first step.
Understand the QMS requirements ==> Conduct gap analysis ==> Establish plan
Below is a section from our Planning Guide mentioned earlier and available from our website. So again if you would like to download go to our website and its available at no charge.
????
Take a look at these articles to further guide you in your QMS journey
There are many detailed steps in the implementation of the ISO 13485 QMS for a start-up business that may not have a good understanding of the requirements, or experienced resources of their own to carry out the implementation. Here are the basic steps we recommend:
- Assign a Project Leader with experience with ISO 13485 or can complete the online training or other external training options. (Note: This is a role we can take on or support)
- Complete a Gap Analysis using our check sheet.
- Develop the Implementation Plan
- Customize the QMS documentation as required and release into the QMS
- Training as required including for ISO 13485:2016 requirements early in the implementation and ongoing for each procedure as they are released.
- Audit the implementation as it progresses and make corrective actions as required.
- Obtain Certification
??????????????????????