
My experience leading the implementation of many quality management systems, and later as a consultant helping start-up medical device companies with their ISO 13485:2016 quality management system implementation, I have continued to be totally convinced that completing a Gap Analysis as part of the Implementation Planning step, is absolutely essential to the success of the project.
As part of the PDCA cycle, taking the time to complete the gap analysis and use the information obtained to help develop the plan for the QMS implementation, will go a long way to ensure you don?t miss any of the ISO 13485:2016 requirements and provide the information needed to determine the resources required to fast-track and complete implementation and certification on time.

So here are the 3 major activities involved in developing the ISO 13485:2016 implementation and certification plan:
1) Learn and understand the requirements included in the ISO 13485:2016 Standard
2) Complete the Gap Analysis
3) Assessment of the findings from the Gap Analysis and develop Implementation Plan
More on these 3 activities:
Learn and understand the requirements included in the ISO 13485:2016 Standard:
I highly recommend you purchase at least one copy of ISO 13485:2016 Medical devices ? Quality management systems ? Requirements for regulatory purposes
There are online resources where you can obtain controlled copies from globally recognized certification bodies. Auditors could also very well ask to see your copy and how you control it.
The goal is to learn the detailed requirements of the ISO 13485:2016 so you can then conduct the Gap Analysis. Here is a summary of the 5 elements of the standard:
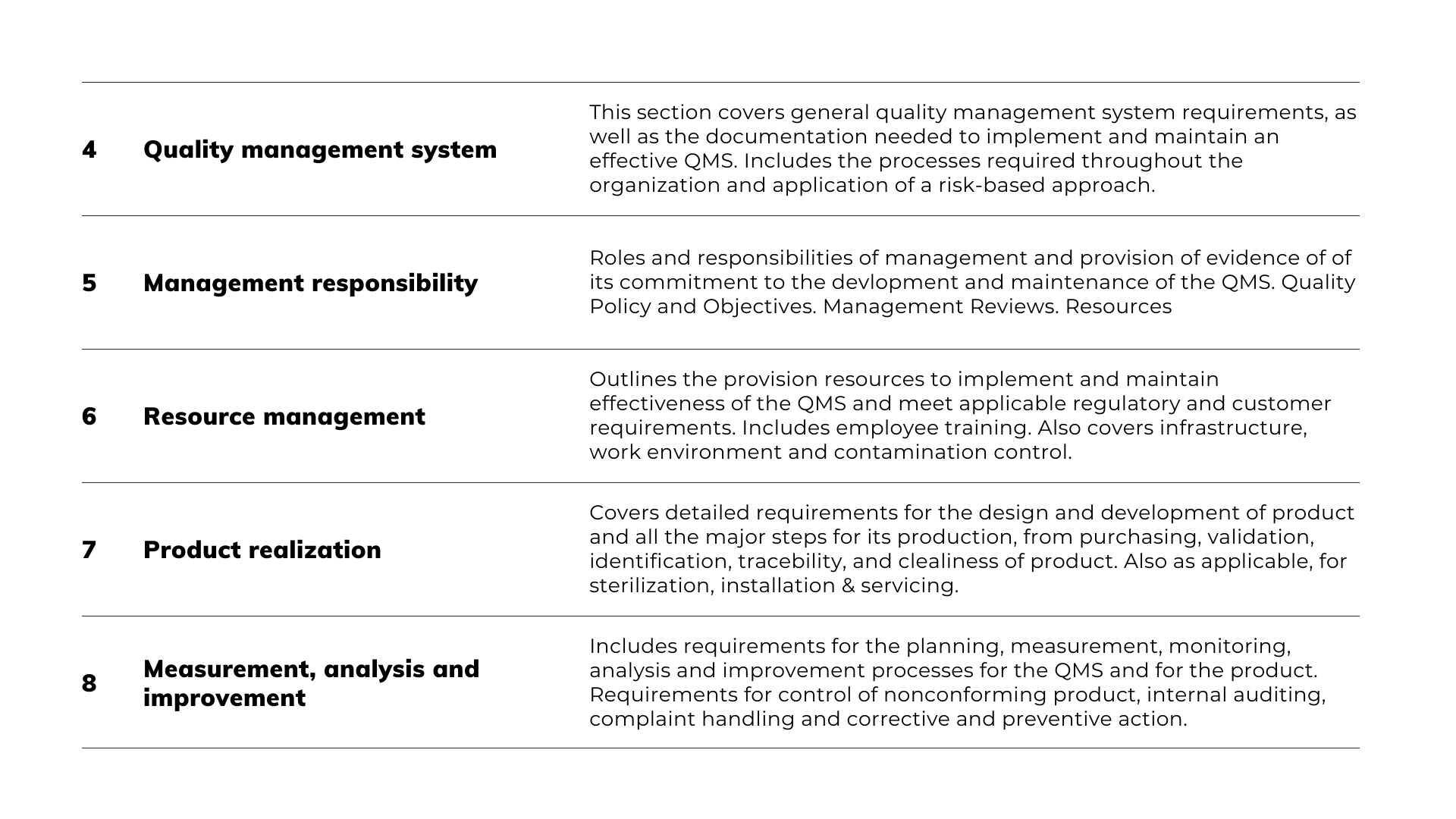
Complete the Gap Analysis
After you have obtained a copy of the Standard and perhaps received some external training if required, you can complete the Gap Analysis Checksheet.
The Gap Analysis Checklist offered by Fast-Track QMS Consultants, is made up of 43 questions to highlight any gaps between the current readiness and the requirements of the 5 elements of the ISO 13485:2016 Standard. There are also some general questions regarding the status the product development as well as manufacturing capability. These can all be factors in establishing the timeline and resources required to design and implement the QMS.
Below is a small example from the Gap Analysis Checklist that covers element 4:
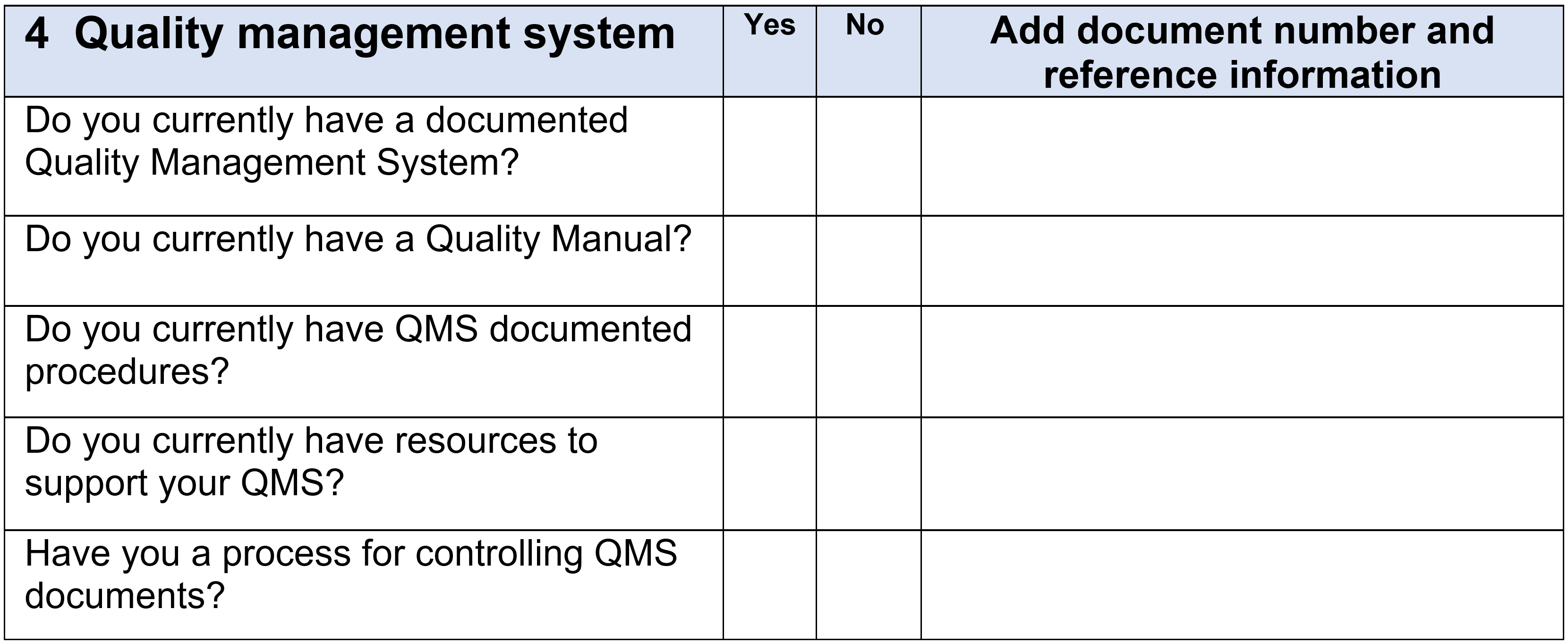
Assessment of the findings from the Gap Analysis and develop Implementation Plan:
So O.K. now you have a good clear understanding of the requirements for each section of the ISO 13485 Standard and the gaps between your current situation and those requirements. This should provide all the information needed to draw up the implementation plan.
Some other questions and potential gaps to consider in the planning include:
- At what stage of development is your medical device and manufacturing capabilities?
- Are resources available , are they trained or have experience with ISO 13485:2016
- What are the intended markets for your medical device, regulatory requirements, etc.
Final Thoughts:
To repeat, the Gap Analysis Checklist is a critical part of the planning process. If you?re a start-up medical device company, and on a fast-track timeline, you don?t have the luxury of missing any requirements of the ISO Standard or finding out mid-way through the project that you did not plan enough resources.
It?s all about starting off with a good Plan before you Do, Check, Act, and that is exactly what the Gap Analysis Checklist provides, is the foundation for your Plan.
At Fast-Track QMS Consultants we have helped many medical device companies conduct this gap analysis and our experience and expertise can provide a robust implementation plan and provide a high degree of confidence that companies will meet their goals for QMS Implementation and Certification.
Fast-Track QMS Consultants offers a free download from our website, a complete Planning Kit which includes a Gap Analysis Checklist.
??To go to our website, cut and paste this link; https://fasttrackiso13485.com/
