Start-up medical device companies can be inclined to put training off to sometime down the road, and after they have developed their device and manufacturing capabilities. There is nothing in the regulatory requirements that specifies when you need to start training, and so for many it can be tempting to only to comply sometime before the certification audits.
This is not however best practice and can cost your more time and headaches later if you take this approach. I truly believe and recommend having a training procedure in place and conducting the appropriate training early on, is the right way to go.
Of course all the required documented procedures for ISO 13485:2016 are important in order to achieve certification and provide the quality system you need but for a start-up there are in my experienced opinion some procedures which should come first and others than can wait a little longer. The training SOP and conducting documented training is one of those.
For more on Priority Procedures and a Complete Guide to ISO 13485:2016 Implementation check out the following guides:
Why is the training procedure such a high priority for start-ups?
?????????
1) Training is a basic tool to produce quality results & get thinks right the first time.
2) As you hire new employees you start off with training and generate records.
3) Helps to build a quality culture
4) Saves time in the long run
5) Helps to mature your training process before certification
Now let?s take a look at the requirements for training for ISO 13485:2016 and the FDA
The Training requirements to meet ISO 13485:2016 Section 6.2 and FDA CFR 21 Part 820.20
ISO 13485:2016: Section 6.2 Human resources states the following:
Personnel performing work affecting product quality shall be competent on the basis of appropriate education, training, skills and experience.
The organization shall document the process(es) for establishing competence, providing needed training, and ensuring awareness of personnel.
The organization shall:
a) determine the necessary competence for personnel performing work affecting product quality;
b) provide training or take other actions to achieve or maintain the necessary competence;
c) evaluate the effectiveness of the actions taken;
d) ensure that its personnel are aware of the relevance and importance of their activities and how they contribute to the achievement of the quality objectives;
e) maintain appropriate records of education, training, skills and experience (see 4.25)
Note: The methodology used to check effectiveness is proportionate to the risk associated with the work for which the training or other action is being provided.
FDA CFR 21 Part 820.20 and 820.25
820.20 (2) Resources, states the following:
Each manufacturer shall provide adequate resources, including the assignment of trained personnel, for management, performance of work, and assessment activities, including internal quality audits, to meet the requirements of this part.
820.25 Personnel, states the following:
(a) General. Each manufacturer shall have sufficient personnel with the necessary education, background, training, and experience to ensure that all activities required by this part are correctly performed.
(b) Training. Each manufacturer shall establish procedures for identifying training needs and ensure that all personnel are trained to adequately perform their assigned responsibilities. Training shall be documented.
(1) As part of their training, personnel shall be made aware of device defects which may occur from the improper performance of their specific jobs.
(2) Personnel who perform verification and validation activities shall be made aware of defects and errors that may be encountered as part of their job functions.
So the training requirements are fairly clear for ISO 13485 and FDA CFR Part 820, but both leave the timing of training up to each company. This then is a temptation for many start-ups to delay training which again I advise against.
So in summary the justification for making training a priority and starting early in your QMS implementation includes:
1. Training as a basic tool to produce quality results:
It?s not exactly rocket science to understand if an employee is trained on their work responsibilities before they start performing their function there is a much higher probability that they will produce quality results. This is true no matter the work they are responsible for, be it design and development of a medical device to loading a container with finished product.
So obviously training of engineers on the design and development procedure and requirements comes before loading the container. As an example, the consequences of not performing risk analysis or validations correctly have the potential for major negative implications both from a product quality and safety perspective as well as regulatory issues.
So in training and development programs provide a lot of benefits. They enhance employee performance, boost employee productivity, major factor in producing quality products, reduce cost of poor quality, and improve the company culture.
2. Training for new employees:
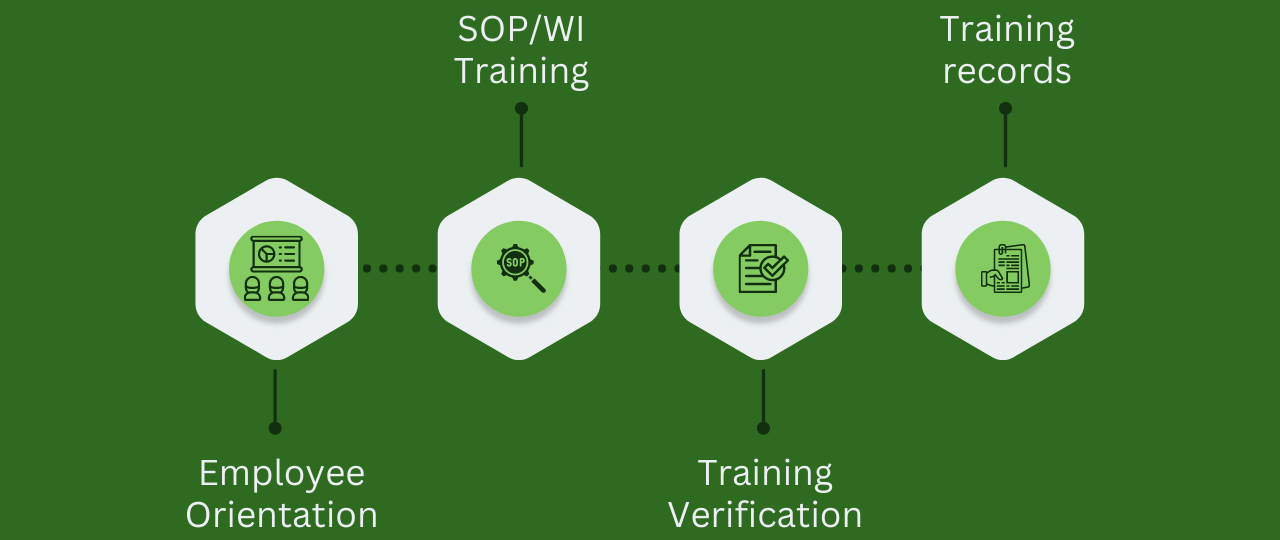
So even though ISO 13485:2016 and the FDA regulations don?t specify when training must take place it is fairly obvious the biggest benefits and lowest risk is for training to take place for new employees and before they start performing their job function. This training can of course be tailored made to suite the job the employee is expected to perform. Normally, and I believe the best practice is for the training process to include as appropriate:
Another major benefit in conducting training early in your QMS implementation and for new employees is you have time for refresher training as required, and an opportunity to improve your training process before certification.
To learn more about Fast-Track QMS Consulting and the products and consulting services we can provide, including training procedure and training materials along with online training as required then just click here